Vaccination against Bluetongue serotype 3 has started in Spain with Syvazul BTV3

Press release
León, Thursday 3rd October 2024
Laboratorios Syva announces that the first doses of its vaccine Syvazul BTV3 already arrived in Andalusia and Extremadura, allowing to start promptly the vaccination programme against this new serotype.
In September 2023, Bluetongue serotype 3 was detected for the first time in the Netherlands, spreading rapidly to neighbouring countries.
In September 2024, outbreaks were detected in the south of Portugal, very close to the border with Spain, which set in motion the protocols for the prevention and control of Bluetongue by the MAPA (Spanish Ministry of Agriculture, Fisheries and Food.) and the Autonomous Communities.
After the MAPA urged the need for vaccines against serotype 3, on 25 September 2024, the AEMPS gave its approval, for the emergency use of Syvazul BTV3 in accordance with Art. 110 (2) of the (EU) 2019/6 Regulation.
Ultimately, on 30 September, the first outbreaks were confirmed in Spain, in the provinces of Huelva and Badajoz.
Laboratorios Syva supplied the first doses to MAPA, with Syvazul BTV 3 being the first vaccine applied immediately for the control of serotype 3 in Spain on October 2, 2024.
Over the next few days, the Autonomous Communities of Castilla y León, Extremadura and Andalusia will receive more doses of Syvazul BTV3 to be able to continue the vaccination programme.
Bluetongue serotype 3 has been particularly aggressive in both sheep and cattle, causing high mortality in sheep and severe clinical signs in cattle.
Vaccination is the most effective method to control Bluetongue.
Syvazul BTV3 is indicated for active immunisation in sheep and cattle, thereby reducing clinical symptoms and viraemia caused by Bluetongue serotype 3.
Syvazul BTV3 is a vaccine against Bluetongue serotype 3 authorised for emergency use in the Netherlands, Belgium, Germany, Austria and now in Spain in accordance with Art. 110 (2) of Regulation (EU) 2019/6. Syvazul BTV3 is also approved for emergency use in the UK. Since the first authorisation, more than 3 million doses have already been administered in these countries.
More information on the disease and on the vaccine on this web page
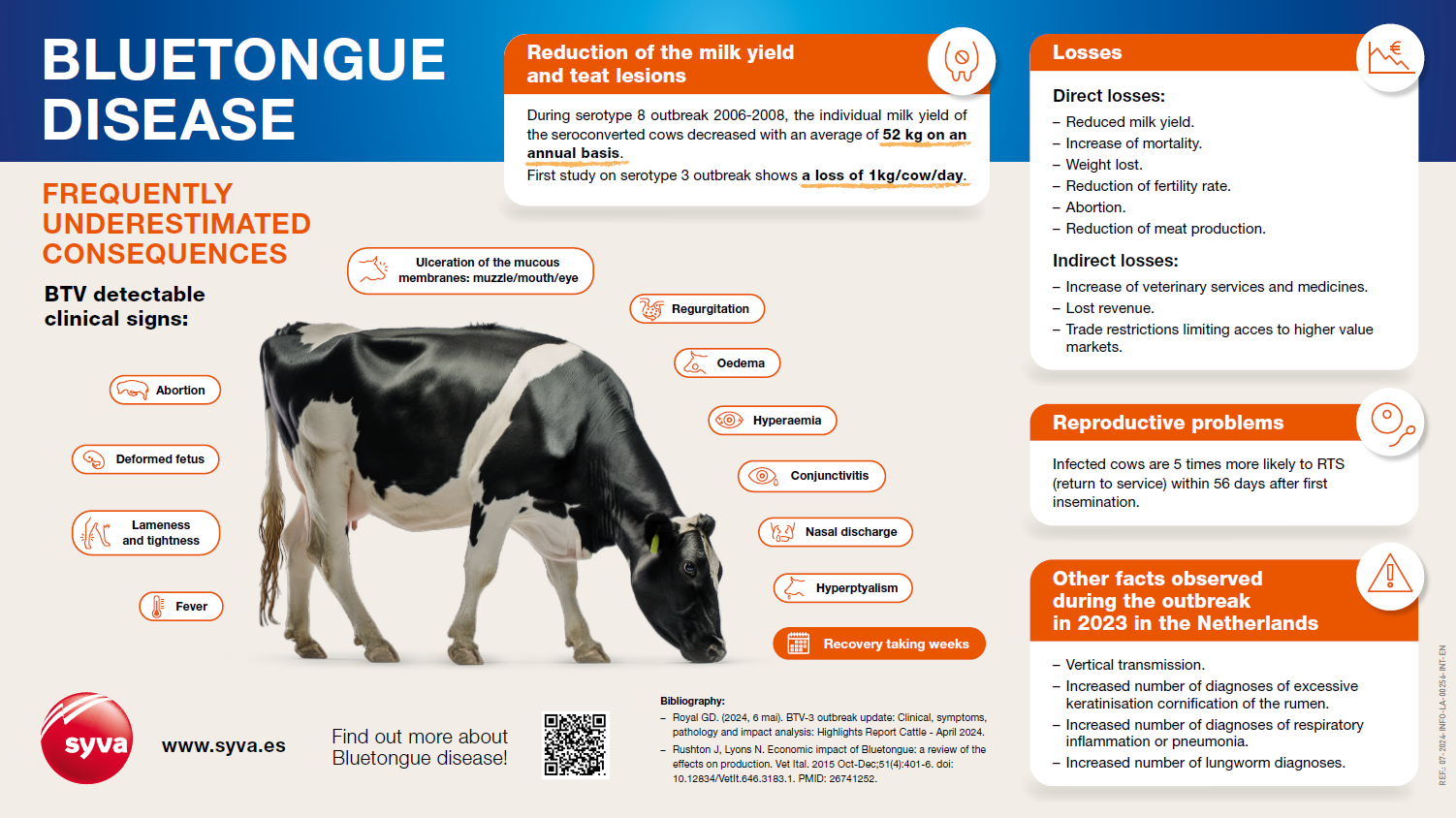
Bluetongue Infographic: don’t underestimate the consequences!
Video over Bluetongue
Downloads
You may also be interested in

30 January, 2025
Syvac® EH Marker, the first and only DIVA vaccine against EHD, is now authorized in Belgium
Syva is pleased to announce that the FAMHP/FAGG has granted emergency use authorization for its marker (DIVA) vaccine against EHD in accordance with Article 110(2) of Regulation (EU) 2019/6 for use in cattle and deer.

24 January, 2025
Call for the 28th edition of the Syva Award International for the best doctoral thesis in Animal Health
We are pleased to remind you that the call for applications for the 28th Syva Award International is open until 18 February 2025. We also remind you that for two years now, the Syva Award International is no longer limited to Spanish candidates, but invites all doctoral students of any nationality who have submitted their thesis in animal health...

22 January, 2025
Syva receives a positive opinion for Syvazul BTV 3 vaccine from the CVMP of the European Medicines Agency (EMA).
On 15 January 2025, the Committee for Medicinal Products for Veterinary Use (CVMP) issued a positive opinion recommending the granting of a marketing authorization for Syvazul BTV 3 vaccine. This step forward marks an important milestone in the fight against Bluetongue, and Syva is proud to be at the forefront of this innovation.



